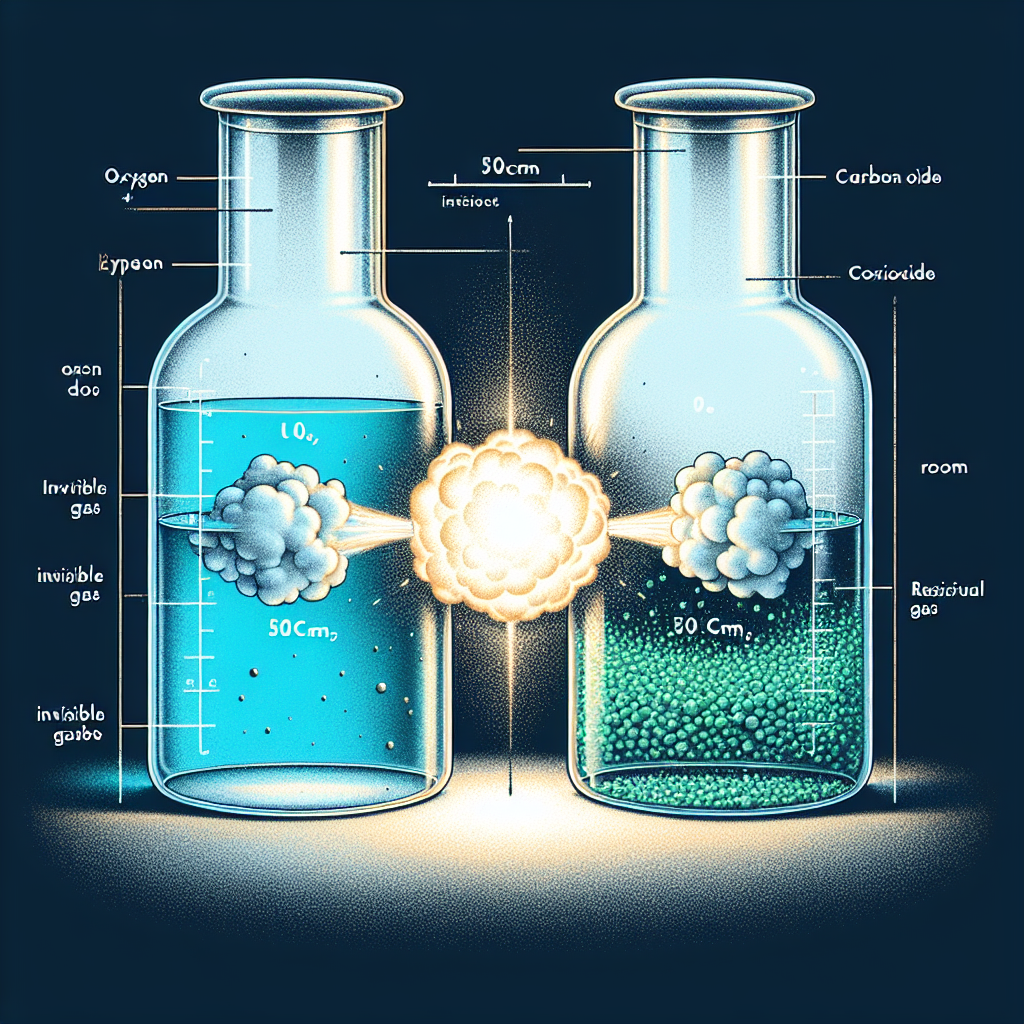
A volumue of 50cm3 of oxygen was exploded in 50cm3 of carbon (ii)oxide .what was the volume of the residual gas at room temperature
2CO + O2 ==> 2CO2
50 cc CO will require 25 cc O2 and will produce 50 cc CO2. That will leave 25 cc O2 remaining as un-reacted. Total volume of gas after reaction will be 75 cc; i.e., 50 cc CO2 + 25 cc O2 un-reacted. I don't know what residual gas means so take your pick of 75 cc total or 25 cc unreacted CO.
Help me with that question
2CO+O2=2CO2
2 divided by 2 is equal to 1.
The reaction between magnesium and nitrogen is represented by the following equation:
3Mg + N2 → Mg3N2
This is a synthesis reaction, where magnesium (Mg) and nitrogen (N2) combine to form magnesium nitride (Mg3N2). The reaction requires high temperatures (above 350 degrees Celsius) and is often carried out under a blanket of inert gas, such as argon, to prevent the formation of magnesium oxide due to exposure to air.
Magnesium nitride is a white solid that has a variety of industrial uses, such as in the production of ceramics, pesticides, and rocket fuels. It can also react with water to form magnesium hydroxide and ammonia gas.
This equation represents the reaction between carbon monoxide (CO) and oxygen (O2) to form carbon dioxide (CO2).
Two molecules of CO react with one molecule of O2 to produce two molecules of CO2. This reaction is exothermic, meaning it produces heat.
It is a common industrial process, often used in the production of steel or in the manufacture of chemicals.